Functional Composite Materials
Different synthesis techniques are used, e.g. precipitation methods, solvo-/hydrothermal reactions, sol-gel techniques, and solid-state routes. Nanocomposites consisting of carbon nanofibers decorated with oxide materials are prepared by electro-spinning.
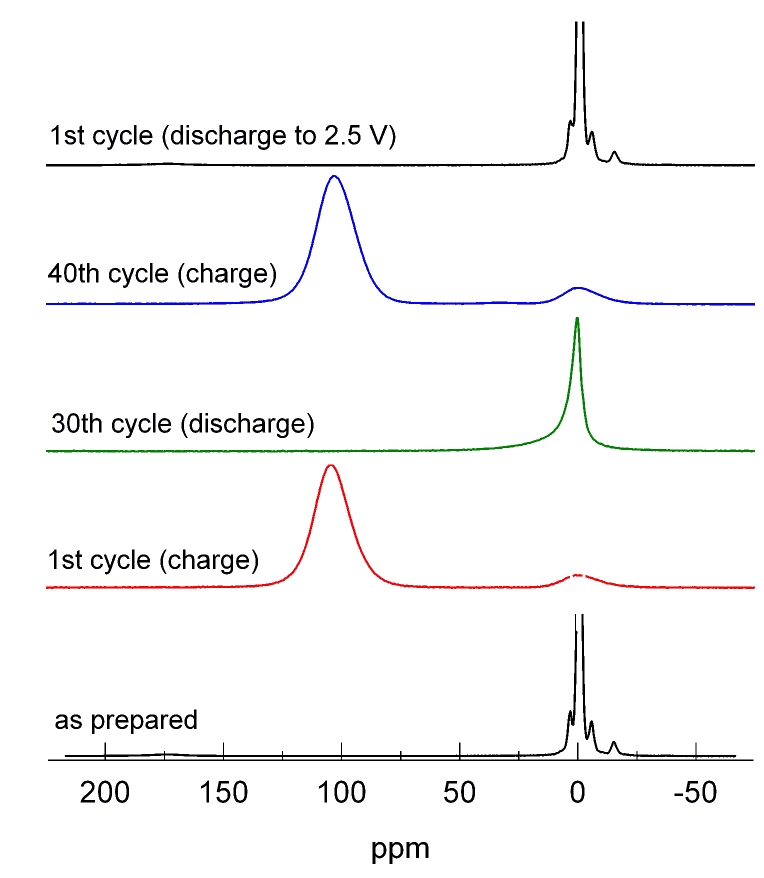
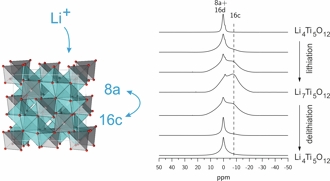
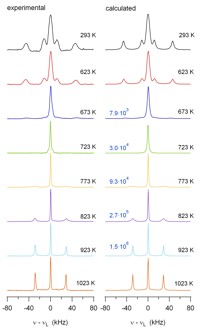
One focus is on rechargeable Li-ion batteries. The aim is to elucidate the electrochemical reaction mechanisms that occur during insertion and removal of Li into/from the electrode materials and that are responsible for function and degradation of the batteries. Besides standard battery testing we use solid-state NMR spectroscopy, Mössbauer spectroscopy, X-ray absorption spectroscopy, and diffraction methods to study the changes in the local/long-range structure of the host materials as well as the mobility of the Li ions. Finally, the results of these fundamental studies are used for optimization of the materials with respect to the battery performance.
Further projects are dealing with the proton and oxygen ion mobility in electrolytes for PEM and SOFC fuel cells, respectively. This mobility is investigated with temperature-dependent 1H/2H and 17O NMR spectroscopy and relaxation time measurements.
[1] N. Schweikert, R. Heinzmann, A. Eichhöfer, H. Hahn, S. Indris, Solid State Ionics 226, 15 (2012).
[2] H. Hain, R. Heinzmann, M. Scheuermann, L. Wünsche, H. Hahn, S. Indris, Solid State Nucl. Magn. Reson. 42, 9 (2012).
[3] S. Indris, P. Heitjans, R. Uecker, B. Roling, J. Phys. Chem. C 116, 14243 (2012).
Electro-spinning
Electrodes for electrochemical devices have to fulfill several functions. First of all, they provide matrices for the active species, e.g. lithium in Li-ion batteries. Secondly, they conduct electrons as well as reaction species allowing the access of reactants and the exit of products. A material, which has been successfully applied as the support material in fuel cells and Li-ion batteries is the electroconducting polymer PANI. However, the effect of support morphology - leaving the chemistry unchanged - on the electrochemical performance has not been investigated in detail, so far.