Carbon based materials
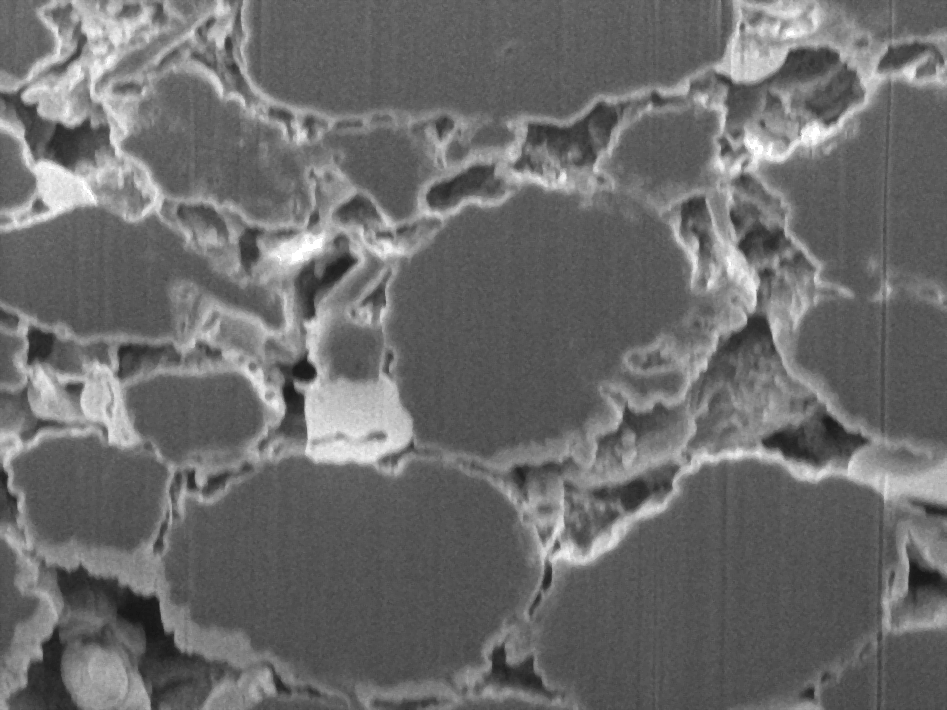
Thanks to the high electron conductivity, low density and chemical inertia the most common use of carbon is that of an electrode additive to improve the overall conductivity. But the 3dimensional network of carbon inside the electrode not only aids electron conduction but also impacts the ionic conduction in the electrolyte phase by defining the porosity and tortuosity of the electrode. Hence, even in such a rather simple application it is essential to understand the complex interplay of the 3D carbon structure, processing parameters and the final electrode morphology.
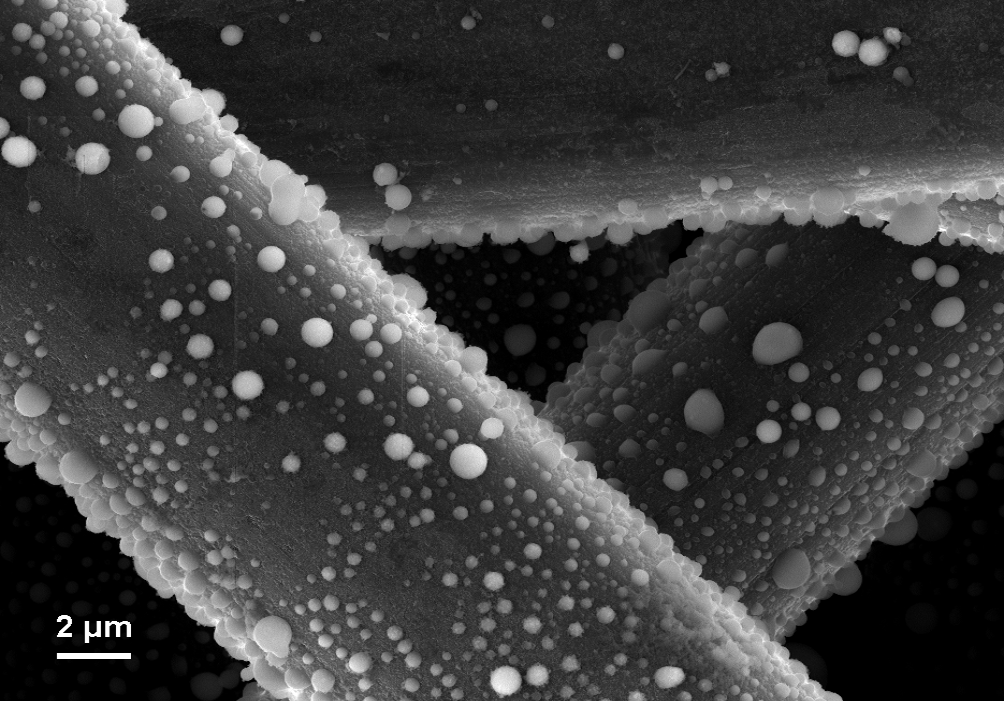
However, the versatility of carbon goes far beyond the simple use as an electrode additive. In lithium-ion-batteries graphite serves as the active anode component, reversibly intercalating up to 372 mA/g of lithium. The intercalation of lithium into graphite involves a sequence of phase changes and is accompanied by complex surface reactions due to the limited stability of the electrolyte. These reactions lead to the formation of a protective layer on the graphite surface, the so called solid-electrolyte-interphase layer (SEI). This layer is essential for the operation and lifetime of a lithium-ion-battery and is therefore intensively studied at the IAM-ESS.
Another application of carbon studied at the IAM-ESS are electrodes for lithium-air and vanadium-redox-flow batteries. In both battery types the interfaces of the carbon electrode play a decisive role for the electrochemical reactions. The structural diversity of carbon materials combined with its rich surface chemistry not only offer a playground for chemists to fine-tune the material properties but are also a challenge to the analysis and elucidation of electrochemical surface processes of carbon electrodes. Therefore, a major aspect of our research involves the development of characterization methods which function as in situ analytical techniques to obtain insight into the complex electrochemical reactions taking place at the electrode interfaces.