Electrolytes and electrochemical Methods
We develop liquid and solid polymer electrolytes for lithium-ion and post-Li battery technologies. In our group electrolytes are tested for their stability and degradation mechanisms in energy storage systems with the aim to improve the battery cycle life and battery safety.
Electrolytes are one of the three primary components in Li- and post-Li batteries. They are responsible for the ionic transport between the two electrode materials, namely anode and cathode that store cations in their host structure. The mechanical and (electro)chemical properties of the electrolyte are paramount for long cycle life and battery safety. The transport properties of ions in a given electrolyte material determine its ionic conductivity and thus the so-called rate-capability of the battery, including its fast charging capabilities.
The aim of our research is to tailor the electrolyte properties for improved safety, to boost durability in the highly demanding environment of high-voltage batteries and to tweak the fast charging capabilities. This requires fundamental understanding of the chemical and physical processes in the material during battery operation and under different operation conditions, for which a broad spectrum of chemical and electrochemical analytical methods is employed.
Electrolytes for Lithium and Post-Li Batteries
Battery aging is a result of decomposition reactions of electrolyte components at the interfaces of anode and cathode, when their electrochemical stability window is exceeded. As a result, charge carriers (like lithium ions) that have contributed previously to the capacity of the battery are permanently lost as they deposit as salts on the electrode surface and thus participate no longer the cell reactions.
This is a particular challenge for so-called post-Li technologies, such as the sodium- or potassium-ion battery, that are supposed to complement current Li-ion technology in fields where scalability and low cost are essential. Unfortunately, decomposition reactions in these systems are more pronounced and for this reason the cycle life of post-Li batteries has so far not reached the same level as for established Li-ion batteries.
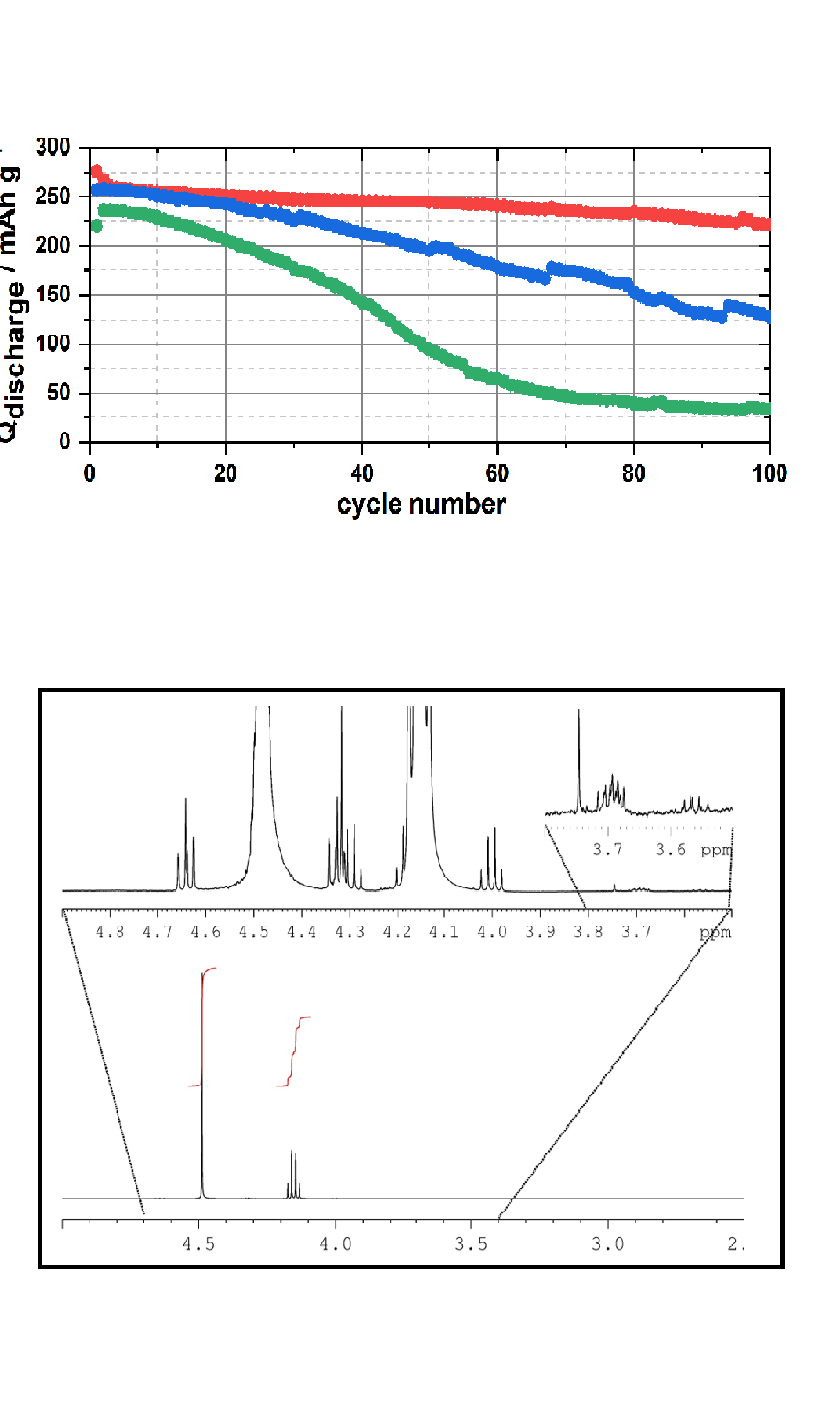
Understanding decomposition mechanisms of electrolyte components is therefore a major objective in our research in order to select suitable materials and additives for next-generation electrolyte mixtures. For this we combine electrochemical characterization techniques (Fig. 1a), with analysis of liquid and solid decomposition products that accumulate either in the electrolyte or on the electrode surface, using nuclear magnetic resonance (NMR) spectroscopy (Fig. 1b), photoelectron spectroscopy (XPS) or electrochemical impedance spectroscopy (EIS). In addition, we apply gas chromatography mass spectrometry (GC-MS) and calorimetric techniques in collaboration with other research groups in the Post-Li Storage Cluster of Excellence (POLiS).
Electrolyte Development: From Liquid to Solid Polymer Electrolytes
Moving from conventional liquid electrolyte mixtures to solid electrolytes is considered an important technological transition from state-of-the-art Li-ion batteries towards next-generation energy storage systems with improved safety properties, yet potentially higher energy densities and longer cycle life.
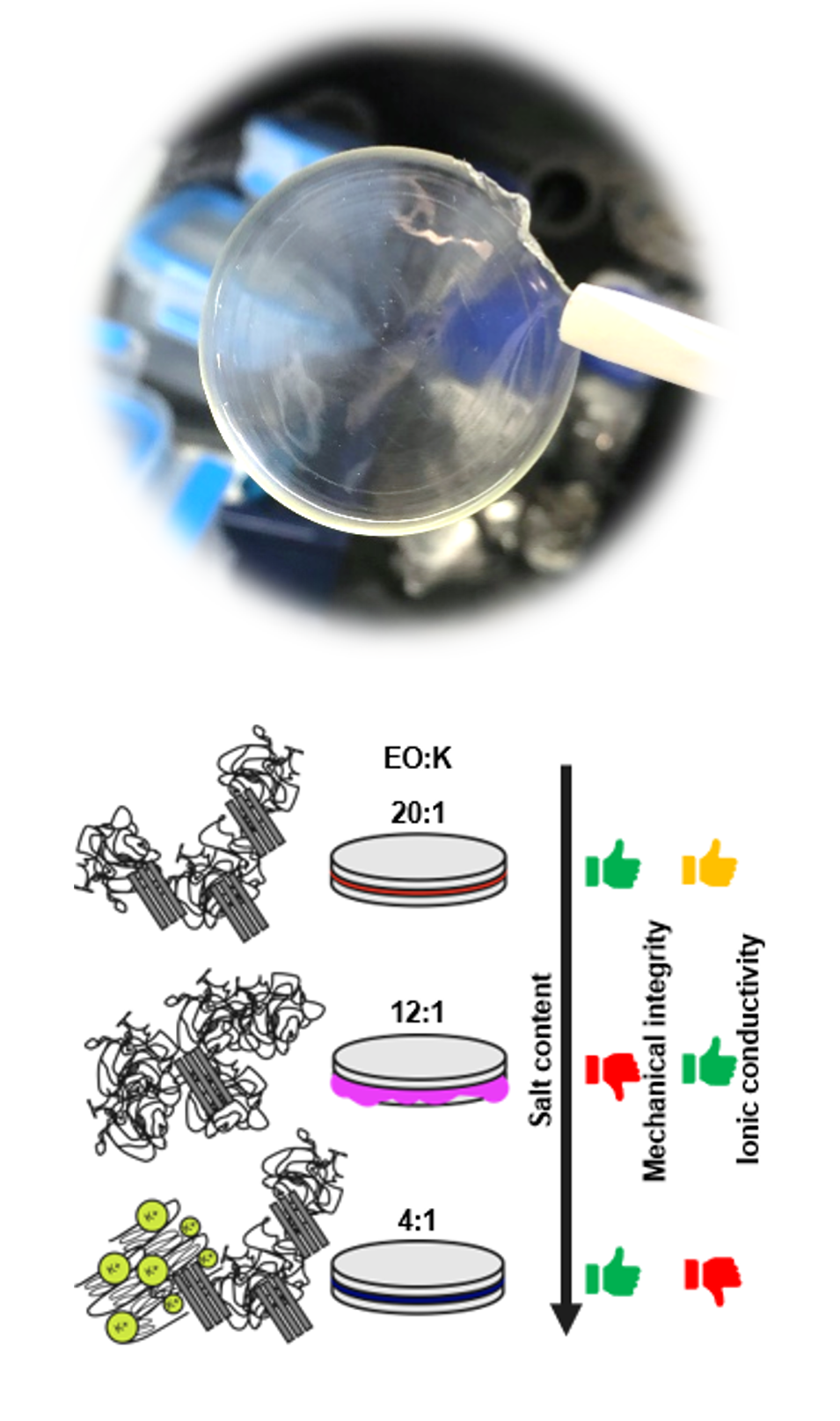
One class of solid electrolytes are polymer electrolytes that dissolve, similar to their liquid counterparts, electrolyte salts in a solid host matrix. Under an applied voltage, i.e. during battery operation, charge carriers can travel through the solid matrix. Polymers exhibit several benign properties compared to liquid electrolytes, including their inherently higher safety (no components with high vapor pressure or low flashpoint; lower toxicity), ease of processability and a broader electrochemical stability window that is generally associated with lower degrees of side reactions and thus better capacity retention. A prominent example of the successful integration of polymer electrolytes into Li-Ion batteries is the Belloré Bluecar.
Our research activities currently focus on polymer electrolytes for potassium-ion batteries. The aim is to improve the ionic conductivity of the materials to a degree that allows room temperature applications, while maintaining good mechanical integrity in a broad temperature range, as well as electrochemical stability in a broad voltage range. The material development is supported by a portfolio of different methods from the field of polymer chemistry and electrochemistry, such as the characterization of rheological, thermal and morphological properties (e.g. using differential scanning calorimetry (DSC)), infrared spectroscopy to investigate polymer-salt interactions, as well as electrochemical characterization techniques, including electrochemical impedance spectroscopy (ionic conductivity) and voltammetric techniques to test for electrochemical stability. The polymer electrolytes are further tested directly in battery configurations for their suitability in actual battery applications.
Ongoing Projects & Activities
- We participate in the Post Lithium Storage Cluster of Excellence (POLiS) (https://www.postlithiumstorage.org/en/science#c4368 )
- Work package C.1: Structure, Function and Morphology at Metal Electrodes
- Work package X.1: High Energy Potassium Batteries
- K-Ion Batteries: Sustainable Strategies (KIBSS) (DFG Projekt #448719339)
- Lithium recovery and battery-grade materials production from European resources (LiCORNE)
- NSERC-DFG SUSTAIN: Prussian White for Sustainable Separation and Purification Technologies (desa-LiNa-te) (DFG Projekt # 533389065)
- Lithium recovery and battery-grade materials production from European resources (LiCORNE, https://www.licorne-project.eu/ ) (Grant Agreement No 101069644)
With friendly support by:



(PhD student)
Sacrifical salts as electrode additives in sodium- and potassium-ion batteries
(PhD student)
Functionalization of Calcium-Metal Interfaces


(PhD Student)
Ion Exchange Materials for Direct Lithium Extraction from Geothermal Brines
(PhD student)
Selective, Electrochemical Lithium Extraction from Lithium-rich Brines
(PhD student)
Degradation at the Graphite-Electrolyte Interface of Automotive Batteries
(Postdoctoral Researcher)
Development of Novel Ion Exchange Materials for the Selective Lithium Extraction from Brines
(Postdoctoral Researcher)
Synthesis of Polyimide-based Polymer Electrolytes for Battery Applications
Publications
Kardeş, G.; Röse, P.; Wildersinn, L.; Korneychuk, S.; Jeschull, F.; Pundt, A.; Grunwaldt, J.-D.; Krewer, U.
2026, January 22. doi:10.35097/vf3j9gx4te0ftpqc
Kardeş, G.; Röse, P.; Wildersinn, L.; Jeschull, F.; Korneychuk, S.; Pundt, A.; Grunwaldt, J.-D.; Krewer, U.
2026. ACS Catalysis, 16 (1), 211–227. doi:10.1021/acscatal.5c03350
Herrmann, L.; Bohn, N.; Pfau, A.; Kölbel, T.; Ehrenberg, H.; Binder, J. R.; Jeschull, F.
2025. ChemSusChem, 18 (13), e202500530. doi:10.1002/cssc.202500530
Johansson, I. L.; Kolesnikov, T. I.; Khudyshkina, A.; Rauska, U.-C.; Brandell, D.; Mindemark, J.; Hernández, G.; Jeschull, F.
2025. Solid State Ionics, 432, 117069. doi:10.1016/j.ssi.2025.117069
Orth, A.; Höfer, H. H.; Wildersinn, L.; Jeschull, F.; Reischl, M.
2025. Current Directions in Biomedical Engineering, 11 (1), 330–333. doi:10.1515/cdbme-2025-0184
Heyn, A.; Röder, C.; Geßwein, H.; Ahmadian, A.; Velazquez-Rizo, M.; Bohn, N.; Jeschull, F.; Binder, J. R.
2025. ChemSusChem, 18 (16), e202501111. doi:10.1002/cssc.202501111
Nitschke, F.; Goldberg, V.; Dashti, A.; Spitzmüller, L.; Jeschull, F.; Stechern, A.; Kohl, T.
2025, June 25. Helmholtz Energy Symposium (2025), Karlsruhe, Germany, June 24–27, 2025
Schöner, S.; Schmidt, D.; Wildersinn, L.; Wolf, S. E.; Speer, S.; Wolff, B.; Bokov, A.; Cao, P.; Windmüller, A.; Chen, X.; Tsai, C.-L.; Jeschull, F.; Tempel, H.; Yu, S.; Eichel, R.-A.
2025. Small Science, 5 (6), 2400655. doi:10.1002/smsc.202400655
Khudyshkina, A.
2025, July 7. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000181673
Rauska, U.-C.; Röder, C.; Kolesnikov, T. I.; Deng, B.; Jeschull, F.
2025. ACS Applied Polymer Materials, 7 (6), 3498–3503. doi:10.1021/acsapm.4c04137
Wildersinn, L.; Stottmeister, D.; Jeschull, F.; Groß, A.; Hofmann, A.
2025. ACS Applied Materials & Interfaces, 17 (6), 10055–10072. doi:10.1021/acsami.4c17461
Panasenko, I.; Bäuerle, M.; Jeschull, F.
2025. Electrochimica Acta, 513, 145551. doi:10.1016/j.electacta.2024.145551
Panasenko, I.
2025, February 24. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000177870
Jeschull, F.; Kataev, E.; Panasenko, I.; Njel, C.; Félix, R.; Maibach, J.
2025. Advanced Energy Materials, 15 (6). doi:10.1002/aenm.202403811
Schmidt, D.; Schöner, S.; Steinhoff, M. K.; Schierholz, R.; Steinhauer, K.; Thomas Daniel, D.; Speer, S.; Kretzschmar, A.; Jeschull, F.; Windmüller, A.; Tsai, C.-L.; Tempel, H.; Yu, S.; Eichel, R.-A.
2024. Small Structures, 5 (12). doi:10.1002/sstr.202400311
Sbrascini, L.; Sarapulova, A.; Gauckler, C.; Gehrlein, L.; Jeschull, F.; Akçay, T.; Mönig, R.; Marinaro, M.; Nobili, F.; Dsoke, S.
2024. Batteries & Supercaps, 7 (12), e202400207. doi:10.1002/batt.202400207
Kolesnikov, T. I.; Voll, D.; Jeschull, F.; Theato, P.
2024. European Polymer Journal, 217, Art.-Nr.: 113315. doi:10.1016/j.eurpolymj.2024.113315
Schöner, S.; Schmidt, D.; Chen, X.; Dzieciol, K.; Schierholz, R.; Cao, P.; Ghamlouche, A.; Jeschull, F.; Windmüller, A.; Tsai, C.-L.; Liao, X.; Kungl, H.; Zhong, G.-M.; Chen, Y.; Tempel, H.; Yu, S.; Eichel, R.-A.
2024. ACS Nano, 18 (27), 17924–17938. doi:10.1021/acsnano.4c04507
Jeschull, F.
2024. ChemElectroChem, Art.-Nr.: 202400254. doi:10.1002/celc.202400254
Xing, S.; Khudyshkina, A.; Rauska, U.-C.; Butzelaar, A. J.; Voll, D.; Theato, P.; Tübke, J.; Jeschull, F.
2024. Journal of The Electrochemical Society, 171 (4), 040516. doi:10.1149/1945-7111/ad3b72
Schäfer, D.; Hankins, K.; Allion, M.; Krewer, U.; Karcher, F.; Derr, L.; Schuster, R.; Maibach, J.; Mück, S.; Kramer, D.; Mönig, R.; Jeschull, F.; Daboss, S.; Philipp, T.; Neusser, G.; Romer, J.; Palanisamy, K.; Kranz, C.; Buchner, F.; Behm, R. J.; Ahmadian, A.; Kübel, C.; Mohammad, I.; Samoson, A.; Witter, R.; Smarsly, B.; Rohnke, M.
2024. Advanced Energy Materials, 14 (15), Art.-Nr.: 2302830. doi:10.1002/aenm.202302830
Jeschull, F.; Hub, C.; Kolesnikov, T. I.; Sundermann, D.; Hernández, G.; Voll, D.; Mindemark, J.; Théato, P.
2023. Advanced Energy Materials. doi:10.1002/aenm.202302745
Stottmeister, D.; Wildersinn, L.; Maibach, J.; Hofmann, A.; Jeschull, F.; Groß, A.
2023. ChemSusChem, 17 (3), Art.Nr.: e202300995. doi:10.1002/cssc.202300995
Khudyshkina, A. D.; Rauska, U.-C.; Butzelaar, A. J.; Hoffmann, M.; Wilhelm, M.; Theato, P.; Jeschull, F.
2024. Batteries and Supercaps, 7 (1), Art.-Nr.: e202300404. doi:10.1002/batt.202300404
Colombo, F.; Müller, M.; Weber, A.; Keim, N.; Jeschull, F.; Bauer, W.; Ehrenberg, H.
2023. Energy Advances, 2, 2093–2108. doi:10.1039/d3ya00246b
Hofmann, A.; Müller, F.; Schöner, S.; Jeschull, F.
2023. Batteries & Supercaps, 6 (12), Art.Nr.: e202300325. doi:10.1002/batt.202300325
Khudyshkina, A. D.; Butzelaar, A. J.; Guo, Y.; Hoffmann, M.; Bergfeldt, T.; Schaller, M.; Indris, S.; Wilhelm, M.; Théato, P.; Jeschull, F.
2023. Electrochimica Acta, 454, Article no: 142421. doi:10.1016/j.electacta.2023.142421
Jeschull, F.; Pham, H. Q.; Ghamlouche, A.; Thakur, P. K.; Trabesinger, S.; Maibach, J.
2023. Journal of Physics: Energy, 5 (2), 025002. doi:10.1088/2515-7655/acbbee
Jeschull, F.; Zhang, L.; Kondracki, Ł.; Scott, F.; Trabesinger, S.
2023. Journal of Physics: Energy, 5 (2), Art.-Nr.: 025003. doi:10.1088/2515-7655/acbbed
Ghamlouche, A.; Müller, M.; Jeschull, F.; Maibach, J.
2022. Journal of The Electrochemical Society, 169 (2), Art.-Nr.: 020541. doi:10.1149/1945-7111/ac4cd3
Smith, A.; Stueble, P.; Leuthner, L.; Hofmann, A.; Jeschull, F.; Mereacre, L.
2023. Batteries & Supercaps, 6 (6), e202300080. doi:10.1002/batt.202300080
Surace, Y.; Jeschull, F.; Novák, P.; Trabesinger, S.
2023. Journal of The Electrochemical Society, 170 (2), Art.-Nr.: 020510. doi:10.1149/1945-7111/acb854
Gehrlein, L.; Leibing, C.; Pfeifer, K.; Jeschull, F.; Balducci, A.; Maibach, J.
2022. Electrochimica Acta, 424, Art.-Nr.: 140642. doi:10.1016/j.electacta.2022.140642
Gehrlein, L.; Njel, C.; Jeschull, F.; Maibach, J.
2022. ACS Applied Energy Materials, 5 (9), 10710–10720. doi:10.1021/acsaem.2c01454
Khudyshkina, A. D.; Morozova, P. A.; Butzelaar, A. J.; Hoffmann, M.; Wilhelm, M.; Theato, P.; Fedotov, S. S.; Jeschull, F.
2022. ACS Applied Polymer Materials, 4 (4), 2734–2746. doi:10.1021/acsapm.2c00014
Allgayer, F.; Maibach, J.; Jeschull, F.
2022. ACS applied energy materials, 5 (1), 1136–1148. doi:10.1021/acsaem.1c03491
Butzelaar, A. J.; Röring, P.; Mach, T. P.; Hoffmann, M.; Jeschull, F.; Wilhelm, M.; Winter, M.; Brunklaus, G.; Théato, P.
2021. ACS applied materials & interfaces, 13 (33), 39257–39270. doi:10.1021/acsami.1c08841
Jeschull, F.; Maibach, J.
2020. Electrochemistry communications, 121, Art.-Nr. 106874. doi:10.1016/j.elecom.2020.106874