The anode materials which operate through reversible conversion mechanism have been brought to interest in the beginning of 21st century due to their intrinsically higher capacity compared to the insertion-type electrodes. Nanocrystalline Iron oxides are especially attractive because of low cost and environmental compatibility. In the scope of the WeNDeLIB project, a comparative study of different metal ferrites as conversion type model system for Li-ion batteries, to elucidate the influence of partial substitution of Fe in the spinel structure with different 3d-cations, is reported. Their electrochemical performance and mechanism involving the electrochemical reaction is investigated using various in situ techniques.
The 3d-transition metal ferrites MFe2O4 (M = Fe, Co, Ni and Cu) are synthesized by a simple and environmental friendly co-precipitation route. The final calcination is performed at different temperatures. The obtained powders are characterized by Scanning Electron Microscopy (SEM) and X-Ray Diffraction (XRD). The structural characterization by XRD confirms the existence of phase pure compounds with spinel structure except for CuFe2O4 and Fe3O4 samples which contain a mixture of different oxides. The electrochemical performance is strongly influenced by the specific 3d-cation, final calcination temperature, electrode composition as well as particle size.
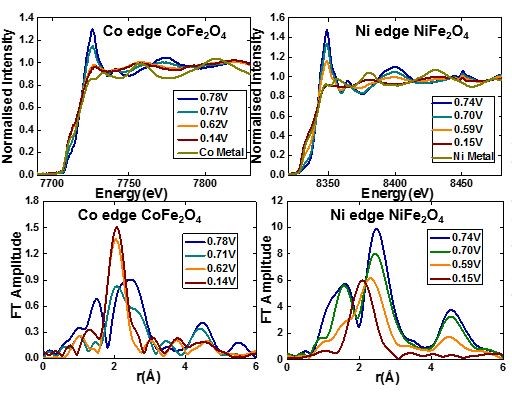
Investigation of nano sized CoFe2O4 and NiFe2O4 using in-situ X-ray absorption reveals the formation of metallic nanoparticles at the end of the first discharge. Reoxidation of Ni and Co was found to be incomplete whereas Fe is completely reoxidized [1].
The electrochemical performance of nano (20-100 nm) and micro (2-5 µm) sized Fe3O4 and CuFe2O4 is also investigated to elucidate the remarkable influence of the specific surface area on the obtainable specific capacities as well as the cycling stability of the electrode materials. Nano sized materials show higher specific capacity in the beginning but there is a drastic fading during cycling, whereas micro sized materials show comparatively lower specific capacity which remains stable over 100 cycles. In order to investigate the electrochemical mechanism in detail, the structural evolution of the materials in the 1st cycle was tracked using in situ synchrotron diffraction and ex situ PDF techniques. It can be observed that the electrochemical mechanism in the first discharge cycle is accompanied by two different processes. Either by the direct reduction of initial material into the respective binary oxide or by a Li-intercalation process in the spinel structure during the initial discharge. This is further transformed to metal nanoparticles occupied in a Li2O matrix.
[1]G. Balachandran, D.Dixon, N. Bramnik, A. Bhaskar, M. Yavuz, L. Pfaffmann, F. Scheiba, S. Mangold, and H. Ehrenberg, Chemelectrochem., DOI: 10.1002/celc.201500197, 2015.
