Neuartige Materialkonzepte
Our research topics focus on the synthesis of advanced functional materials for lithium, post-lithium batteries and supercapacitors. Another important focus is the development and study of electrolytes and interfaces for Aluminium batteries.
Electrode materials for post-lithium batteries
Post-lithium batteries have the potential to store more energy, be safer, and offer a more cost-effective, long-term option for mass applications such as stationary and mobile electrochemical storage. As part of the Cluster of Excellence, POLiS (https://www.postlithiumstorage.org/en/), our group is involved in the synthesis of electrode materials for Na and Mg batteries.
Some of the Na-ion batteries materials under investigation in our group are:
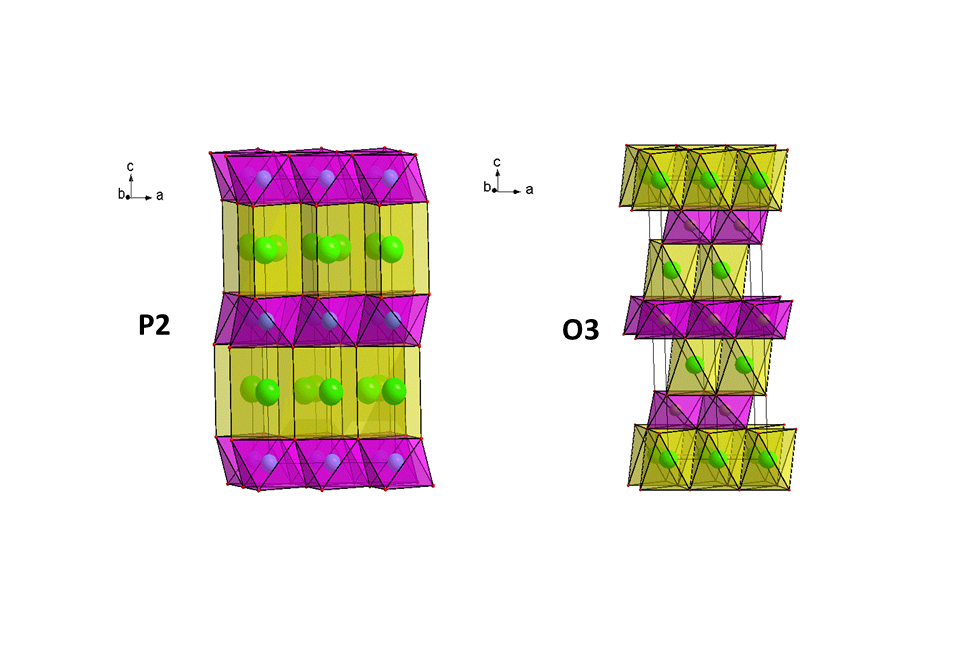
- Compositions, which are isostructural to layered P2-type Na2/3Fe1/2Mn1/2O2 and O3-type NaFe1/2Mn1/2O2 (Fig.1). The 3d-metal cation substitution is applied to obtain new compositions like Na2/3Ni1/6Cu1/6Mn2/3O2 and Na2/3Ni1/3Mn1/2Ti1/6O2. Substituted compositions show improved electrochemical performance with better cycling stability.
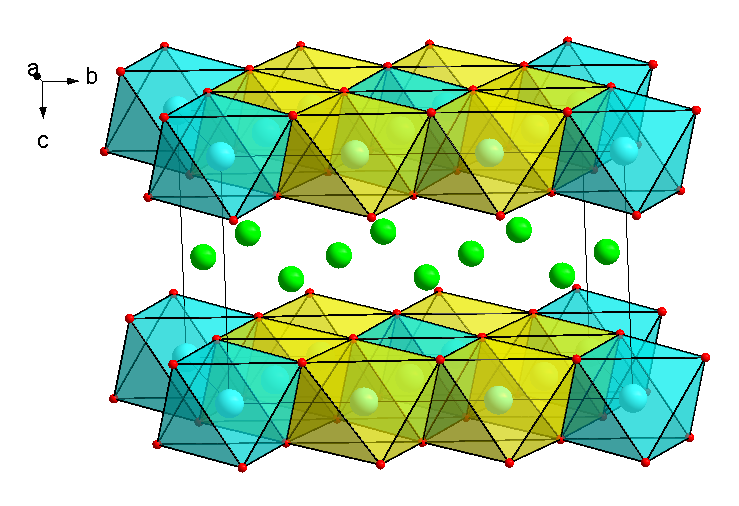
- O3 honeycomb-ordered compositions Na3M2BiO6 and Na3M2SbO6 with M = Ni, Cr, Cu, Co, etc. (Fig. 2). It is interesting to explore the possibility of substitution of Bi5+ with smaller and lighter Nb5+, V5+ ions in this type of structure. Also, a combination of two-valence ions can change the ordering in the 3d-metal plains and hence amend the electrochemistry.
- Conversion and alloy-type materials, like Sb, Sb2O3, FeS, MoS2. These types of materials, used as negative electrodes, deliver a very high capacity in comparison to carbonaceous-type materials. However, during cycling, they are subjected to massive expansion and contraction, resulting in material pulverization and severe loss of performance. In our team, we are looking at different strategies to minimize these effects and improve performance and cycling stability.
In the field of Mg batteries, we focus on the synthesis of positive electrodes such as:
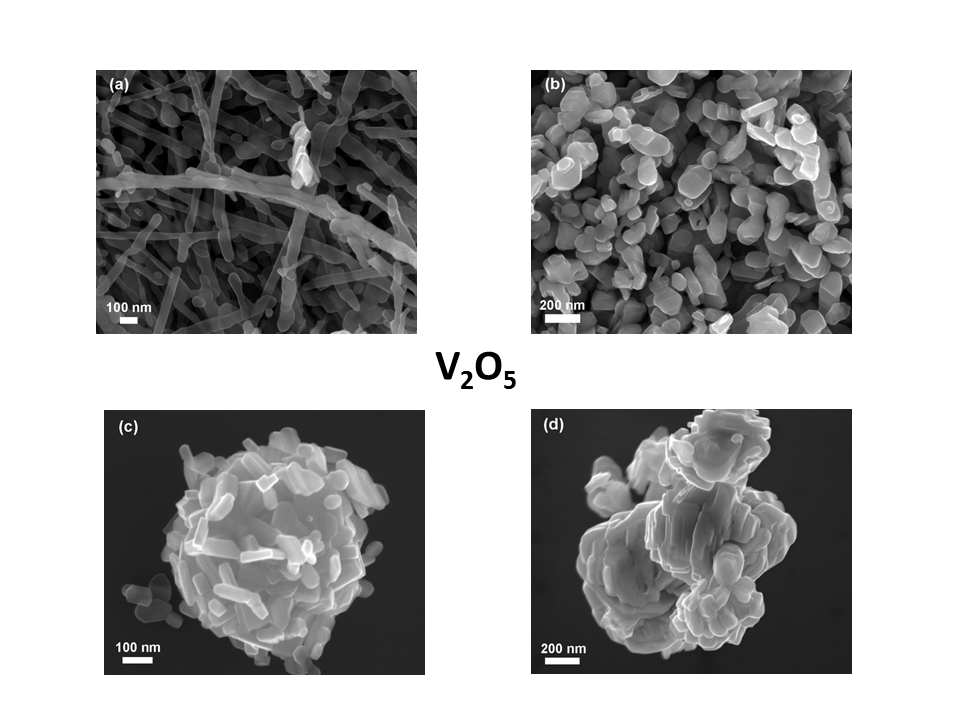
- V2O5 synthesized with different nanoparticle morphology, using the hydrothermal method with various precursors (Fig. 3). We are making correlations between particle shape and electrochemical performance. In addition, we perform doping of MxV2-xO5 with M cations having different oxidation states MxV2-xO5 (M=Cu, Ni, Co, Fe, Ti, Mn) x=0.02, 0.04, 0.06, x=0.1, 0.2.
- Mg batteries synthesized at high pressure conditions
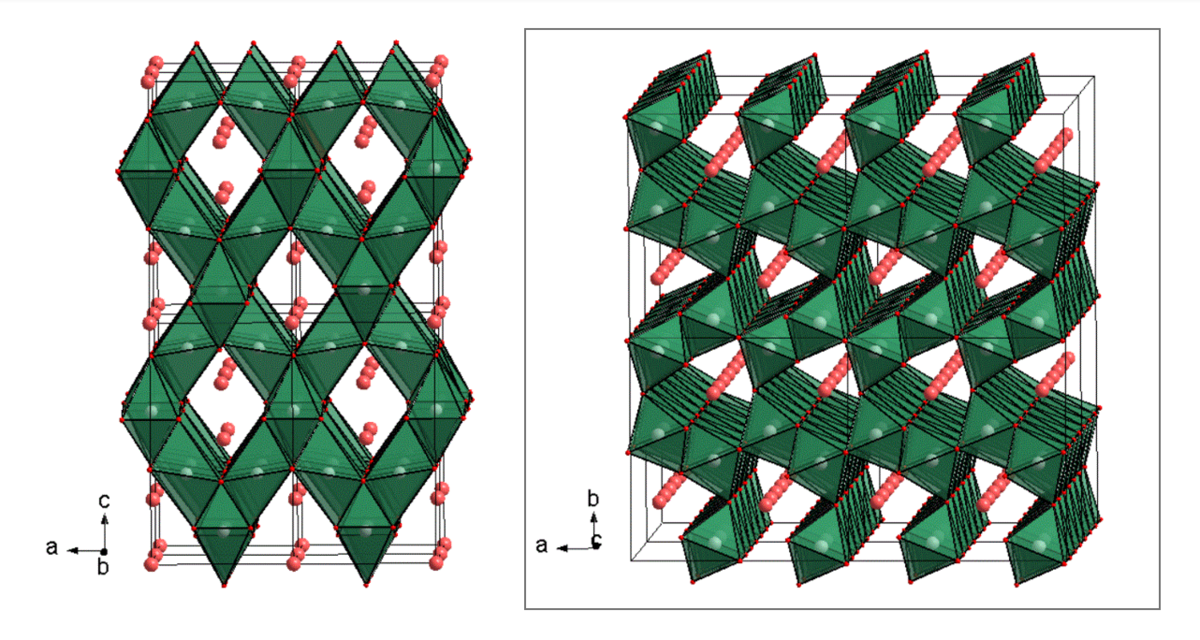
High pressure conditions can lead to structural transformations of host compounds, remaining stable after being quenched. Because of their denser structures, they were excluded as potential battery materials for a long time. But these phase transformations can offer new migration paths for cations, not accessible at ambient pressures. One promising group of compounds is built up by the post-spinel phases, with one of the three well-known forms ofCaFe2O4-, CaMn2O4- or CaTi2O4- type (Fig. 4). First principle calculations predict especially for compositions in the CaFe2O4-type high cationic mobility for divalent ions with astonishingly low migration energy barriers.
One attempt should be made to synthesize Mg-post-spinel compositions using a Walker-type multi-anvil press to investigate the suitability as electrode materials. Especially the 3d transition metal elements Ti, V, Cr on B3+ sublattice are of great interest.
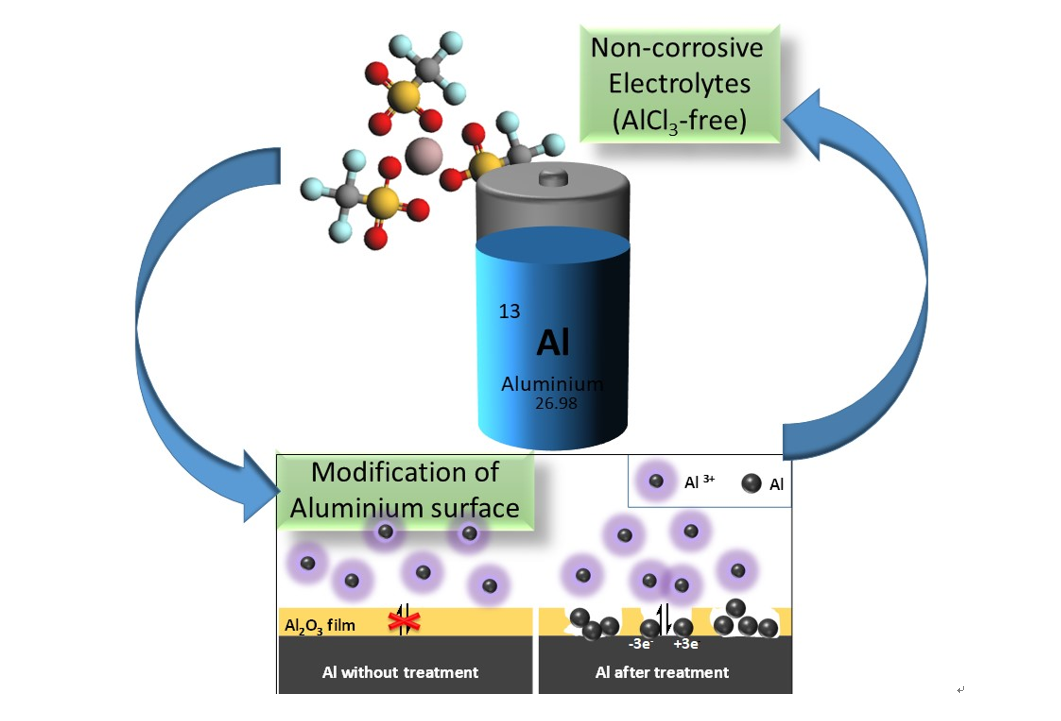
New project granted in POLiS “Towards haloaluminate-free Aluminum batteries”
The aim of this project, in the framework of the Cluster of Excellence (POLiS), is to push the research frontiers on Aluminum batteries by developing a system based on non-corrosive electrolytes and on tailored interfaces. So far, an indispensable component of Al-based electrolytes is AlCl3, which provides a very corrosive environment. This represents a serious obstacle to the development of Al-batteries in terms of sustainability, safety and cost. In this project, alternative Aluminum salts will be explored in combination with deep eutectic solvents. On the other side, the Aluminum surface will be tailored by surface modifications in order to facilitate the Aluminum plating and stripping (Fig.5).
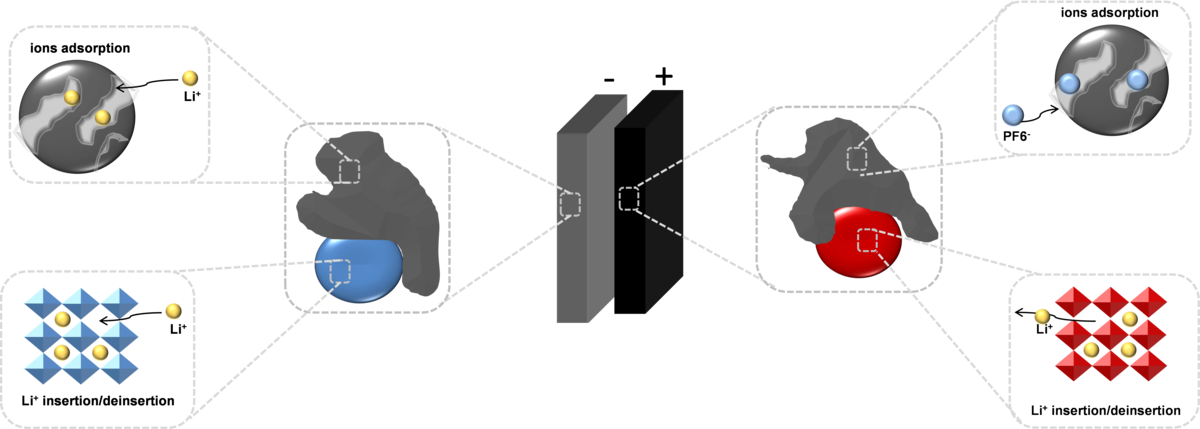
Hybrid and asymmetric supercapacitors
To meet growing demands for electric automotive and regenerative energy storage applications, researchers all over the world have sought to increase the power density of batteries and the energy density of electrochemical capacitors. Hybridizing battery capacitor electrodes can overcome the energy density limitation of the conventional electrochemical capacitors because they employ both the system of a battery-like (redox) and a capacitor-like (double-layer) electrode, producing a larger working voltage and capacitance. Our goal is to hybridize materials at a molecular level by incorporating the battery-like material with a carbonaceous capacitor-type material (Fig.6).